Pharmaceutical And Surgical
IRCLASS Systems and Solutions Pvt Ltd (ISSPL) Lab is NABL accredited laboratory provides testing for a wide range of pharmaceutical products starting from raw materials, excipients, Active Pharmaceutical Ingredients (APIs), bulk drug, intermediates, parenteral preparations, medical devices & surgical items as per Indian and International Pharmacopeia Standards (IP, EP, BP, USP, JP etc.).
Quality control is one of the pre-requisites to regulatory compliances for pharmaceutical products. We have 21 CFR part 11 compliance software for data integrity. Laboratory is well equipped with best technologies like HPLC, AAS, GC, ICP-MS, GC-MS, FTIR, UV Spectrophotometer, LC-MS/MS and IC etc
Inquiry

Our comprehensive range of services under testing for drugs & pharmaceutical products include
- Identification
- Physicochemical Testing
- Assay
- Uniformity of Content
- Method Validation
- Particulate Matter
- Microbiological Analysis
- Sterility
- BET
- Extractable Leachable Analysis
Stability Testing
Stability testing for drugs provides real-time response data of a drug substance or product in the given period under the influence of various environmental factors, such as temperature, humidity and light for long term / intermediate / accelerated storage condition. This data helps to determine & validate the shelf life of pharmaceutical products with respect to its labelled specifications and recommended storage conditions. The drug testing laboratory of ISSPL Lab is well-equipped to conduct stability testing, both real- time and accelerated, as per standards in a wide range of pharmaceutical products. We are performing stability studies as per ICH guidelines (Q1A(R2)) for new drug substances and products.


Extractable and Leachable Studies
Extractable are any element or compounds that can be removed from a packaging compounds or devices, or manufacturing process contact material, into solvents under controlled laboratory conditions. Leachable is an extractable that migrate into a drug product under storage condition. ISSPL can provide customized protocol driven extricable and leachable testing services to ensure that product meets regulatory requirements and compliances
Method Development
We have extensive experience in developing analytical methods using our sophisticated facility for pharmaceutical industries. Some of the applications are
- Quality control of APIs, starting material, intermediates, finished products, impurities, and degradation products
- Stability testing
- Identification and characterization of substances, impurities and degradation products
- Microbiological contaminants determination
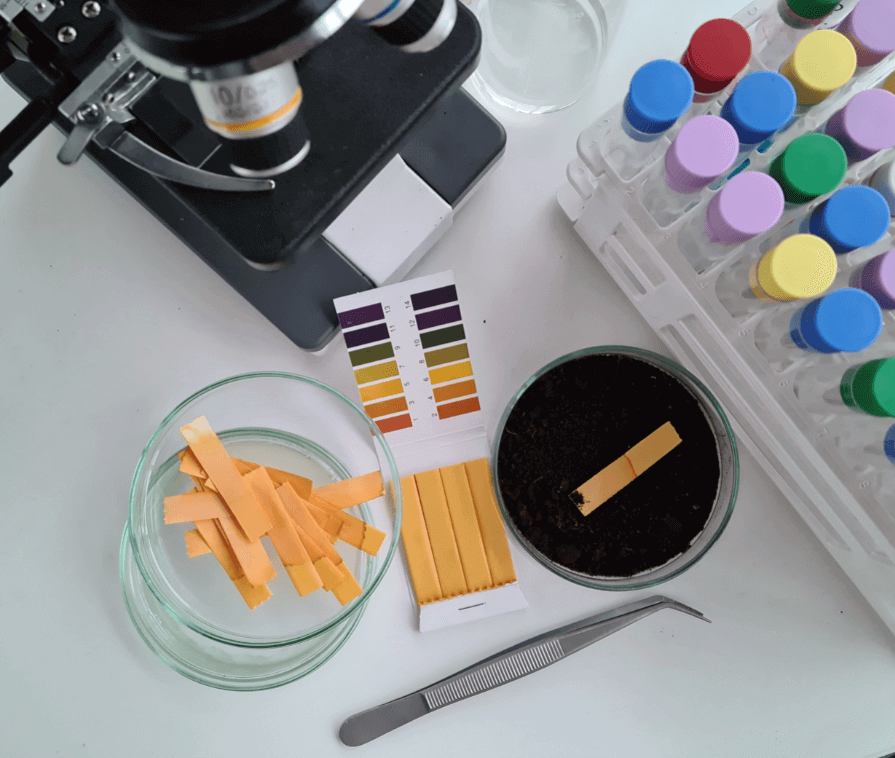

Method Validation
Validation of analytical protocols used in the pharmaceutical industry is critical to ensure that they are suitable, reproducible and ideal for the analysis being carried out. ISSPL Lab’s drug testing laboratory has excelled in performing chemical and microbiological method validations across many industry techniques and product types. We are equipped to assist through the entire process comprising planning, preparation of the protocol, and analytical testing leading to the final project report. The vital areas in which we have expertise include
- Dissolution profile studies
- Product formulation Methodologies
- Stability Testing as per ICH guidelines
Microbiological Studies
Microbial contamination is a significant risk within the pharmaceutical industry, having a potential impact on product integrity and patient safety. Through its well equipped drug testing laboratory, We offers a comprehensive range of microbiological testing services compliant with GMP (Good Manufacturing Practices) requirements for pharmaceutical products, which include
- Sterility Tests
- Bacterial Endotoxin Tests (BET)
- Efficacy Tests
- Microbiological Assays in Antibiotics

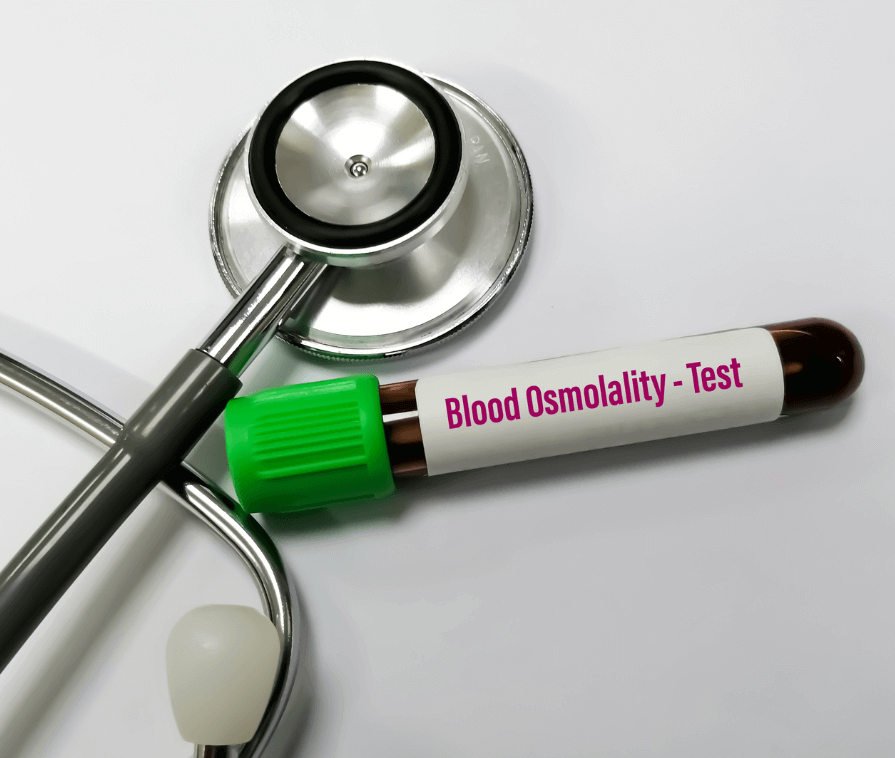
Physicochemical Testing
Physicochemical testing is vital for verifying the quality of manufactured pharmaceutical products before a lot releases into the market. Under physical & chemical parameters, a drug should comply with the specifications mentioned in the latest pharmacopoeia determining its stability, solubility and in-vivo delivery. ISSPL Lab drug testing laboratory is continuously delivering its best analytical services to meet manufacturing and quality standards. Some of the tests we perform under physicochemical testing are
- Appearance
- pH
- Moisture
- Content
- Osmolality
- Viscosity
- Optical activity
- Spectral analysis
Related Substance/ Impurity Testing
The identification of pharmaceutical-related substance or impurity is an essential analytical activity in the drug development process. Pharmaceutical impurities can arise from different sources and significantly damage the purity, stability and safety of drug substances or finished drug products. These impurities/ related substances are generally present at very low levels. Consequently, sensitive and specific assay methods are required to quantify them and collate relevant information and facilitate efficient drug manufacturing processes. ISSPL drug testing laboratory proposes strategies for identifying such impurities/ related substances using sophisticated instruments such as: LC-MS, GC-MS, HPLC, AAS, ICP-MS
We test all product categories, including API & raw materials, and finished products.
Surgical Items
- Tablets
- Capsules
- Injections- Large Volume Parental & Small Volume Parental
- Syrup
- Suspension
- Eye Drops
- Ear Drops
- Ointment
Surgical Items
- Catheters
- Urine Bag
- Gloves
- Masks
- Bandage/Gauge
- Needle
- Sutures
- Adhesive Tape
- Sanitary Napkin
- IV Set
- Cannula
- BT (Blood Transfusion) Set

OUR ACCREDITATION AND RECOGNITION









OUR FEW PRESTIGIOUS CLIENTS
















WHY CHOOSE US
- State-of-the-art testing Lab facility.
- Commitment to quality, safety & integrity.
- One-stop solution.
- Lab has latest equipment and technology.
- Strong customer focus
- Reliability & accuracy.
- Competent, qualified, and experienced technical staff.
- Trusted brand.
- Compliance with quality & safety.
- Presence across all industries.
- Global presence & Pan India operations.
- Pan India sampling team & branch labs.
- Faster turnaround times.
- Dedicated customer care.
ENQUIRY
Testimonials
Frequently Asked Questions
Pharmaceutical Testing Laboratory in India | ISSPL LAB
ISSPL is specialized in drugs and pharmaceuticals testing. medical devices & surgical items. We are one of the best testing labs in India.
In Vitro Permeation Testing - Industry-Leading IVPT Services
ISSPL Testing Lab Has a Large Bank of 120+ Vertical Franz Diffusion Cells to Support IVPT Studies. Work Together with the IVPT Experts at Tergus on Your Topical Semi-solid Project.
Top Pharmaceutical Testing Laboratories in Mumbai - फार्मास्यूटिकल टेस्टिंग लैबोरेट्रीज, मुंबई near me
Pharmaceutical Testing Laboratories in Mumbai. Find ✓Laboratory Testing Services For Water, ✓Laboratory Testing For Food, ✓Laboratory Testing Services, ✓Laboratory Testing For Chemicals, ✓Testing Laboratories in Mumbai. Get Phone Numbers, Address, Reviews, Photos, Maps , FAQs for top Pharmaceutical Testing Laboratories near me in Mumbai
Top Pharmaceutical Testing Laboratories in Chembur East, Mumbai - फार्मास्यूटिकल टेस्टिंग लैबोरेट्रीज, चेम्बूर ईस्ट , मुंबई near me
Pharmaceutical Testing Laboratories in Chembur East, Mumbai. Find ✓Laboratory Testing For Food, ✓Laboratory Testing Services For Water, ✓Laboratory Testing Services, ✓Pathology Labs, ✓Diagnostic Centres in Chembur East, Mumbai. Get Phone Numbers, Address, Reviews, Photos, Maps , FAQs for top Pharmaceutical Testing Laboratories near me in Chembur East, Mumbai
What is pharmaceutical testing?
Pharmaceutical testing involves measuring the accuracy, strengths, and weaknesses of a medical product, determining its potency, efficacy, and proper use or dosage. A pharmaceutical testing laboratory can help you test your products to ensure that they are safe to use by everyone before launching them to the market.
What is a pharmaceutical laboratory?
Pharma Labs means laboratories located within pharmaceutical or biotechnology companies utilized by their own researchers to conduct internal development, clinical research or clinical trials.
Pharmaceutical Testing, laboratories and Research Services
ISSPL Lab offers pharmaceutical testing of raw materials, finished products and research services for approval, developing and manufacturing pharmaceuticals.
Find details on our extensive pharmaceutical and biopharmaceutical tests, including packaging, extractables & leachables (E&L), and toxicology.
Full-service research & development and commercial pharmaceutical testing, method development and validation services.
Pharmaceutical Product Testing Laboratory in India
Pharmaceutical Products Testing | Pharma Testing Laboratory
Material analysis, DSC analysis, chemical tests, physical characterization, NMR testing, FTIR testing, and other procedures can be carried out by testing laboratories in accordance with the specifications and safety protocols provided by the FDA and CDSCO.
Pharmaceutical Testing Laboratories Mumbai, Drug Stability India
Highest Quality Testing. Best Safety Standards. Accurate Results. Contact Us Today! We Offer Specialized Analytical Services At Best Price. Contact Now! Govt. Approved Lab.
Partner with our GMP-compliant CMO for reliable aseptic filling & finish services. Providing Production & Analytical Services. Call ISSPl Labs Today. Formulation Development.
All industries developing products, such as medicines and medical devices and equipment, should first conduct pharmaceutical testing to ensure safe use.
What is QA testing in pharmaceutical industry?
Pharmaceutical Quality Assurance is the assurance of quality requirements for a product or service in the pharmaceutical industry. Quality assurance aims to create and maintain customer confidence in the product, and the goal is to detect defects early or to prevent them.
Related searches- analytical testing laboratories in mumbai, food testing labs in mumbai, pharmaceutical laboratory near me, raw material testing in pharmaceuticals, list of quality control tests in pharmaceutical industry, testing of pharmaceutical products, herbal testing laboratory, analytical labs in navi mumbai,
Top Analytical Laboratories in India
Cities we cater Lab Testing Servives in India: Pharmaceutical Testing Laboratory in Gurugram, Pharmaceutical Testing Laboratory in Haryana, Pharmaceutical Testing Laboratory in Panaji, Pharmaceutical Testing Laboratory in Goa, Pharmaceutical Testing Laboratory in Madurai, Pharmaceutical Testing Laboratory in Mysore, Pharmaceutical Testing Laboratory in Karnataka, Pharmaceutical Testing Laboratory in Amritsar, Pharmaceutical Testing Laboratory in Punjab, Pharmaceutical Testing Laboratory in Jaisalmer, Pharmaceutical Testing Laboratory in Rajasthan, Pharmaceutical Testing Laboratory in Hyderabad, Pharmaceutical Testing Laboratory in Telangana, Pharmaceutical Testing Laboratory in Pune, Pharmaceutical Testing Laboratory in Maharashtra, Pharmaceutical Testing Laboratory in Udaipur, Pharmaceutical Testing Laboratory in Rajasthan, Pharmaceutical Testing Laboratory in Chennai, Pharmaceutical Testing Laboratory in Tamil Nadu, Pharmaceutical Testing Laboratory in Kochi, Pharmaceutical Testing Laboratory in Kerala, Pharmaceutical Testing Laboratory in Varanasi, Pharmaceutical Testing Laboratory in Uttar Pradesh, Pharmaceutical Testing Laboratory in Kolkata, Pharmaceutical Testing Laboratory in West Bengal, Pharmaceutical Testing Laboratory in Bangalore,Pharmaceutical Testing Laboratory in Karnataka, Pharmaceutical Testing Laboratory in Agra, Pharmaceutical Testing Laboratory in Uttar Pradesh, Pharmaceutical Testing Laboratory in Mumbai, Pharmaceutical Testing Laboratory in Navi Mumbai, Pharmaceutical Testing Laboratory in Thane, Pharmaceutical Testing Laboratory in Kalyan, Pharmaceutical Testing Laboratory in Maharashtra, Pharmaceutical Testing Laboratory in Jaipur, Pharmaceutical Testing Laboratory in Rajasthan, Pharmaceutical Testing Laboratory in Delhi
Central Drugs Testing Laboratory (CDTL Mumbai)
Central Drugs Testing Laboratory (CDTL) Chennai , Tamil Nadu. Central Drug Testing Laboratory is one of the Seven National Laboratories in India
What is a drug test laboratory?
Overview. Drug testing laboratories certified by the Department of Health and Human Services receive urine specimens and test them to determine the presence of drugs. They also conduct validity testing to determine if the specimen has been adulterated or substituted.
Which labs are available for drug testing in India?
There are seven Central Drug Testing Laboratories under CDSCO. These are at Kolkata, Mumbai, Chennai, Guwahati, Chandigarh, Kasauli and Hyderabad.
Top Laboratory Testing For Drugs in Mumbai - लेबोरेटरी टेस्टिंग फॉर ड्रग्स, मुंबई - Best Drug Testing Services near me
Laboratory Testing For Drugs in Mumbai. Find ✓Laboratory Testing Services For Water, ✓Laboratory Testing For Food, ✓Laboratory Testing For Chemicals, ✓Laboratory Testing Services, ✓Testing Laboratories in Mumbai. Get Phone Numbers, Address, Reviews, Photos, Maps , FAQs for top Laboratory Testing For Drugs near me in Mumbai
Drug Testing Services, Drug Testing Work in Mumbai, ड्रग टेस्टिंग सर्विस, मुंबई
Drug Testing Services Providers in Mumbai, ड्रग टेस्टिंग सर्विस सर्विस प्रोवाइडर, मुंबई, Maharashtra. Get contact details and address of Drug Testing Services, Drug Testing Work firms and companies in Mumbai
How long is urine good for a drug test?
Do not keep it for longer than 24 hours. The bacteria in the urine sample can multiply if it is not kept in a fridge. If this happens, it could affect the test results. Some sample containers contain preservative so that urine can be stored for longer at room temperature.
Related searches - Pharmaceutical Testing Laboratorylist of drug testing lab in india with its functionPharmaceutical Testing Laboratorylist of central drug laboratory in indiaPharmaceutical Testing Laboratorycentral drug testing laboratory is located atPharmaceutical Testing Laboratorypurpose of drug testing lab in indiaPharmaceutical Testing Laboratorycentral drug laboratory pdfPharmaceutical Testing Laboratorycentral drug laboratory is located at kolkataPharmaceutical Testing Laboratorycentral drug laboratory slidesharePharmaceutical Testing Laboratorycentral drug testing laboratory hyderabad
